Recently, Prof. Bao Siqin and Prof. Li Xihe has cooperated with the research team of Prof. Tang Fuchou of Peking University and published in English version of Science China Life Sciences the article titled “Blastoids generated purely from embryonic stem cells both in mice and humans”. In the research, the researchers developed a one-step method under one added factor (CHIR99021) simple culture condition that successfully induced lineage differentiation and self-assembly of mouse ESCs to form blastoid in vitro.
Three stem cell lines can be derived from blastocysts, i.e. embryonic stem cells(ESCs) from inner cell mass(ICM), trophoblast stem cells (TSCs) from trophectoderm (TE) and extra-embryonic endoderm cells (XENs) from primitive endoderm (PE). Recently, it has been reported that primitive endoderm stem cells(PrESCs) have been successfully formed. In 2018, some research team formed blastoids, self-assembled blastocyst-like structures by a combination of ESCs and TSCs. Blastoids have wide application prospect in organ transplantation and clinic treatment of diseases. To increase the success rate of blastoid formation and its similarity with the features of blastocysts, some reports showed that the stem cells of mice and humans could also be differentiated in vitro to generate complicated blastoid structures with optimized culture media and combination of three cell lines, ESCs, TSCs, and extra-embryonic endoderm cells (XENs), or novel type of stem cells. However, it is unclear whether solely using ESCs can support blastoid generation.
In the research, the researchers used Oct4-ΔPE-GFP (GOF/GFP) mouse ESCs (mESCs) cultured in 2i/L (CHIR99021 and PD0325901, and leukemia inhibitory factor, LIF) media. It was found that when mESCs were only treated with CHIR99021 (CH), the cells exhibited signs of differentiation, the GOF/GFP-labeled mESCs partially differentiated into different types of flattened cell subpopulations, and these differentiated cells were GOF/GFP negative.
The researchers performed 10× Genomics scRNA-seq for CH-induced mESCs. They sequenced 9,676 cells, including 1,403 epiblast (EPI)-like cells, 7,910 primitive endoderm (PE)-like cells, and 363 intermediate (IM) state cells. Compared with previously published ESCs (2i/L cultured) scRNA-seq data (2,684 ESCs),it is showed that the cells treated with CHIR99021 (CH) have three different transcription states, which respectively express EPI markers Pou5f1, Nanog, Sox2, and Klf2, PE markers Gata4, Sox17, and Foxa2 and TE markers S100a11, Id2, Lgals1and Cdx2. To further confirm the differentiation ability of CH, the researchers performed immunostaining tests on CH-treated mESCs. It was revealed that epiblast markers (OCT4 and NANOG), PE marker (SOX17), and TE maker (CDX2) were strongly expressed in the CH-treated mESCs. These results suggest that the mESCs partially differentiated into three lineages of blastocysts in a chemically defined culture system.
Therefore, the researchers further investigated whether the CH-treated mESCs could self-assemble to form the blastoids. To test this hypothesis, they seeded CH-treated ESCs into low-adherence plates. After 3–4 days of incubation, they successfully obtained blastoids and the efficiency of blastoid formation was 26.8%. With the comparative analysis, the researchers found the total cell number of mouse blastoids was similar to that of E4.5 blastocysts while the average diameters of the blastoids were smaller than those of E4.5 blastocysts and the number of inner cell mass (ICM)-like cells was less than that of E4.5 blastocysts. And the results of immunofluorescence staining showed that the blastoids expressed three characteristic lineage markers of blastocysts.
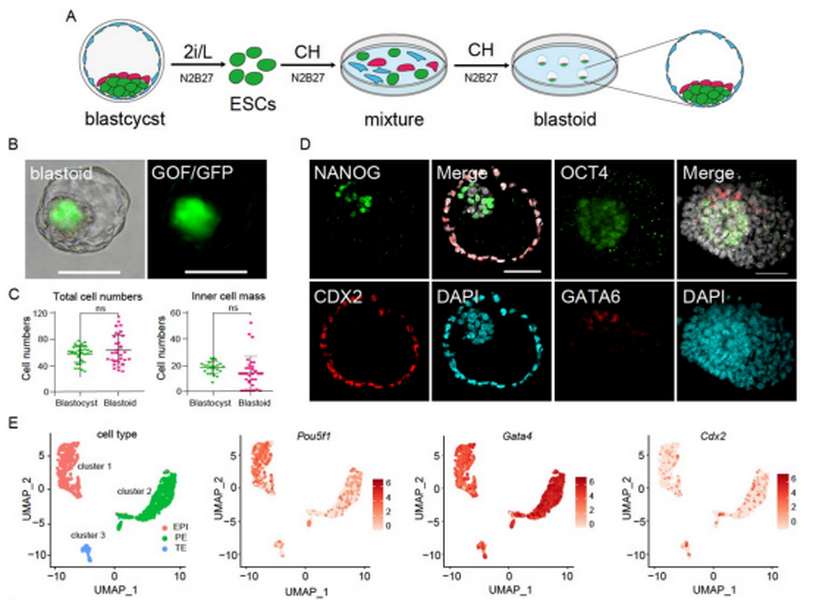
To further test the implantation potency of blastoids, the researchers transferred day 4–5 blastoids into 2.5 days post coitus (dpc) pseudopregnant mice and observed implantation sites at E6.5 (5.4%) and E7.5 (3.3%) in the uteri of the pseudopregnant mice, which was confirmed by immunostaining. The results suggested that these sites could initiate implantation. The researchers also performed scRNA-seq analysis and tested 11 blastoids and 968 individual cells. The results showed that blastoid cells were distributed in three independent clusters, respectively expressing three lineage marker genes of blastocysts. Collectively, these results demonstrated CH-induced self-assembly of mESC into blastoids in vitro.
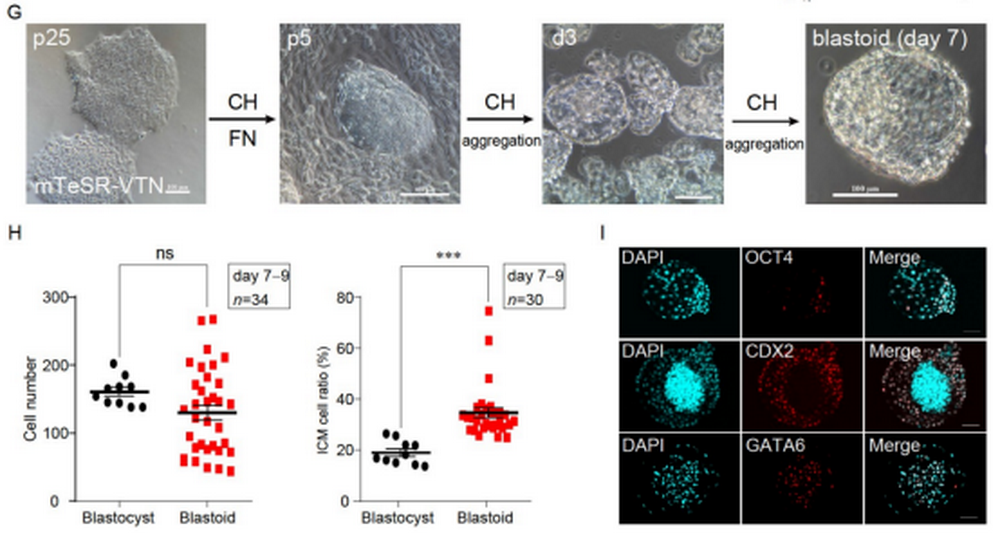
Next, the researchers investigated whether CH can induce the formation of human ESC (hESC)-derived blastoids by employing primed state hESCs. By employing the same culture mejdia and method, they obtained human blastoid(hblastoid) and the efficiency of hblastoid formation in CH conditions was 22.04%. With the test in terms of morphology, size, cell number, and spatial organization of three different cell lineages, it was found that hblastoids resembled human balstcysts. To sum up, the researchers established, in the research, an induction system in a chemically defined medium with only one factor (GSK3 inhibitor CH) added to build blastoids from ESCs not only in mice but also in humans.
Wu Baojiang, special-term associate research fellow of IMU, Wang Yanqiu, PhD student of IMU and Yan Jia and Liu Mengya, PhD students of Peking University are the co-first authors. Prof. Bao Siqin, Prof. Li Xihe of IMU and Prof. Tang Fuchou with Peking University are the co-corresponding authors.
This research was supported by the National Natural Science Foundation of China (32060176), the National Key Research and Development Program of China (2022YFD1302202/3), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT23091), the Program of Higher-Level Talents of Inner Mongolia University (10000-21311201/058), the Inner Mongolia Autonomous Region Natural Science Foundation (2021MS03003), the Inner Mongolia Autonomous Region Science and Technology Plan of China (2020ZD0007/8), the Inner Mongolia Engineering Technology Research Centre of Germplasm Resources Conservation and Utilization (21400-222526).
It is worth mentioning that the research team Prof. Bao Siqin, Prof. Li Xihe and Special-Term Associate Research Fellow Wu Baojiang has published their research results about blastoids where mouse embryonic stem cells are from and transition mechanism of mouse embryonic stem cells from pluripotency to totipotency in Cell Proliferation which is listed among the top journals in the first section of Chinese Academy of Sciences-indexed journals respectively in January and August in 2023.(URL of the articles: https://doi.org/10.1111/cpr.13534;https://doi.org/10.1111/cpr.13396)

Wu Baojiang, special-term associate research fellow of IMU and Wang Yanqiu, PhD student of IMU and Wu Baojiang, special-term associate research fellow of IMU and Yang Zhiqing, PhD student of IMU, are respectively first authors of two articles. And Wu Baojiang, Prof. Bao Siqin and Prof. Li Xihe who are with IMU and Prof. Liu Jun with Northwest Agriculture and Forestry University are respectively the corresponding authors of two articles.
Please log onto the website of Cell Proliferation to read the full texts of two articles.
1. Wu, B., Wang, Y., Yan, J., Liu, M., Li, X., Tang, F., and Bao, S. (2023). Blastoids generated purely from embryonic stem cells both in mice and humans. Sci China Life Sci 66, https://doi.org/10.1007/s11427-023-2419-9.
2. Wu B, Wang Y, Wei X, Zhang J, Wu J, Cao G, Zhang Y, Liu J, Li X, Bao S. (2023). NELFA and BCL2 induce the 2C-like state in mouse embryonic stem cells in a chemically defined medium. Cell Prolif. 2023 Aug 17:e13534. doi: 10.1111/cpr.13534.
3. Wu B, Yang Z, Liu Y, Li J, Chen C, Li X, Bao S. (2023). A chemically defined system supports two distinct types of stem cell from a single blastocyst and their self-assembly to generate blastoid. Cell Prolif. 2023 Jun;56(6):e13396. doi: 10.1111/cpr.13396.